"人体植入血流防感染医用工具"国策研发成功。
페이지 정보
작성자 ㈜MST 조회1,022 View 작성일 25-03-13 12:12본문
**参与“植入式医疗器械”国家研发项目**
MST(KOREA) CO., LTD.(MST·董事长:文雄植·[www.BioCleanAct.com](http://www.BioCleanAct.com)·品牌名:BioCleanAct™)中央研究所被选为**区域特色产业培育+(R&D)区域明星企业培育项目**,并已执行**为期两年的国家研发项目(2023–2024)。**
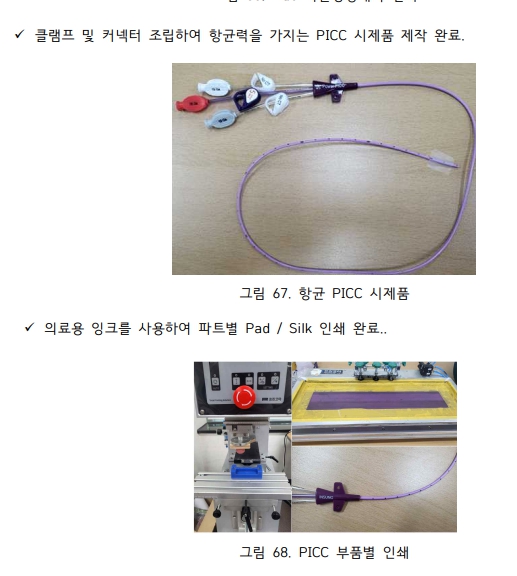
**[开发基于天然合成TPU抗菌复合材料的5Fr、325psi高耐压型防止血流感染的外周置入中心静脉导管(PICC)]**
MST成功完成并推进了**应用天然抗菌复合材料的无感染植入式PICC产品商业化的国家研发项目。**
此外,MST的**新型天然抗菌材料**(用于防止细菌二次感染)已与以下公司在医疗设备领域开展联合研发合作:
- 日本库拉雷集团医疗本部
- 德国西门子医疗
- 韩国三星电子医疗事业部
- 世运医疗(Sewoon Medical)等
**Participation in the National R&D Project for "Implantable Medical Devices"**
MST(KOREA) CO., LTD. (MST, Chairman: Woongsig Moon, [www.BioCleanAct.com](http://www.BioCleanAct.com), Brand: BioCleanAct™) Central Research Institute was selected for the **Regional Specialized Industry Promotion + (R&D) Regional Star Enterprise Promotion Project** and has been conducting a **national research and development project for two years (2023–2024).**
**[Development of a high-pressure 5Fr, 325psi peripherally inserted central catheter (PICC) for the prevention of bloodstream infections using a natural compound-based TPU antibacterial compound]**
MST successfully carried out and completed a **national R&D commercialization project** for an **Infection-free implantable PICC** incorporating its **natural compound-based antibacterial material.**
Furthermore, MST's **new natural antibacterial material** (for secondary bacterial infection prevention) has been the subject of joint research and development in the medical device sector with:
- Kuraray Group Medical Division (Japan)
- Siemens Healthineers (Germany)
- Samsung Electronics Medical Division
- Sewoon Medical, and others.

첨부파일
- MST 뉴스업로드용-250313.pdf (455.8K) 64 Download | DATE : 2025-03-13 12:12:04
- 최종평가결과통지서-250516.pdf (266.6K) 47 Download | DATE : 2025-05-17 12:00:37


