- Nontoxic to the human body
- Admirable anti-bacterial effect
- Broad anti-bacterial activities
- High resistance to the heat and good processing property
- Free from artificial endocrine disruptor
- Functional cosmetic raw materials of Natural product base(premium level)
- Disinfectant, medicine, sanitation products of Natural product base
- Infection free new material for natural product-based human insertion medical device
- Development of new drugs based on natural products
- Anti microbial master batch &
Medical grade nonwoven &
Medical grade textile
Infection free new material for natural product-based human insertion medical device
- Brand name
- BioCleanAct™
- Product code name
- BCA-9002 and others.
- Product feature
- New material added to natural material-based infection free medical equipment
- Product purpose
- About 0.3~0.5% of our antibacterial materials are used in silicone, polyurethane, PVC, and rubber product, which are raw materials for the production of products that require human insertion or secondary bacterial infection of surgical medical equipment and hospital supplies, or require infection free, are properly blended to produce medical equipment through extrusion, injection and molding process.
Product introduction
BioCleanAct™, the world's first new concept bio-friendly natural bio-new material, is mixed with silicone, polyurethane, PVC, and rubber product etc., and extruded, injected and molded to completely solve serious problems such as secondary bacterial infection in the human body, which is new antimicrobial material applied to Bionic Medical Devices for the human body insertion.
- Anti-microbial catheter for a brain and a spinal cord
- Anti-microbial foley catheter
- Medical appliances for a surgery
- Artificial hands and legs made of silicon for the disabled
- Ventilating tube for an eardrum
- Tubes including an endoscope inner tube
- Bite plate
- Medical solution bag (Free from environmental endocrine disruptors)
- Other medical devices
- Kuraray Medical Co., Ltd.
- Biometrix Inc.
- Intercom-Research Inc.
- Samsung Healthcare Inc.
- Siemens Healthcare Inc.
- Joint research with Seoul National University hospital
- Joint research a BK21 project R&D with Inha University
- ISO 9001. (Accredited by Australia & New Zealand)
- Approval of the testing institute (KOTRIC) of Korea FDA.
(KOTRIC : Korea Testing and Research Institute for Chemical Industry) - National R&D Project : Insertion medical catheter of infection-free
- Secure Korean and International patents.

- Product code
- BCA-9002
- Application
- Extrude and inject silicon, polyurethane, PVC and rubber with 0.3 ~ 0.5% of BCA-9002 upon manufacturing medical devices.
- Packing unit
- 10kg, 20kg

This is a catheter appliance which helps to become normal by preventing possible infections and helping the circulation of CFS into brain and spinal cord by draining out of the dura mater using anti-microbial EVD catheter when outbreaking brain edema, brain tumor, head trauma, intraventricular hemorrhage, subarchnoid hemorrhage, intradural and epidural hemorrhage.
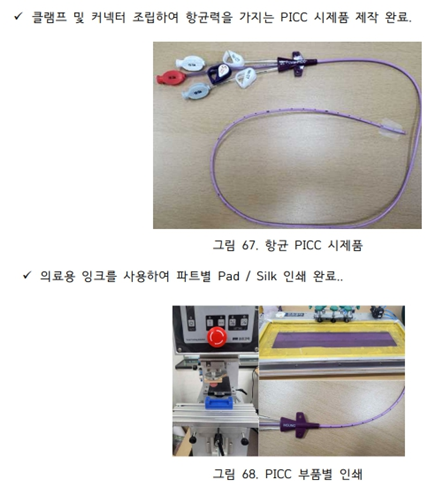

This is a tube catheter for urology which has 2 outlet with 180 degree and a pocket which can change into a balloon in the one end piece, also a Y-type rubber tube having urinating tube and expanding tube which can expand balloon by injecting distilled water.

- Test method
- Culture strain on the surface of the EVD catheter for 24 hours after filling culture fluid in a test tube
- Test strain
- Staphyllococcus epidermis
- Test results
-
- SAMPLE 1Ordinary EVD catheter

- The strain on the surface of the EVD catheter spreads out with pollution into the culture fluid along the direction of the arrow.
- SAMPLE 2Anti-microbial EVD catheter with BioCleanAct™

- EVD catheter with BioCleanAct™, non-toxic anti-microbial material, maintains cleanness same as the initial condition without pollution different from Sample 1 by repressing the strain on the surface in the culture fluid with anti-microbial effctiveness in itself.
- Test method
- Culture foley catheter for 24 hours in the petri-dish after cutting in slice.
- Test strain
- Pseudomonas aeruginosa
- Test results

- SAMPLE 1 : Ordinary foley catheter (Big pollution damage caused by strain)
- SAMPLE 2 : The Foley catheter with BioCleanAct™ (Repress strain of circumference as well as surface of catheter by excellent anti-microbial effectiveness.)






